32nkt

The Current Status Of Galaxy Formation Joe Silk Gary A Mamon
Www Astro Umd Edu Richard Astro421 21 Gas Lec2 18 Pdf
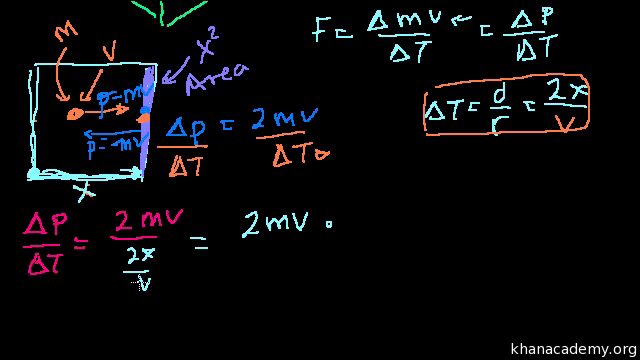
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy

Internal Energy Ideal Gas Monatomic Diatomic Gas Nuclear Power Net
2

First Law Of Thermodynamics With Phet Youtube
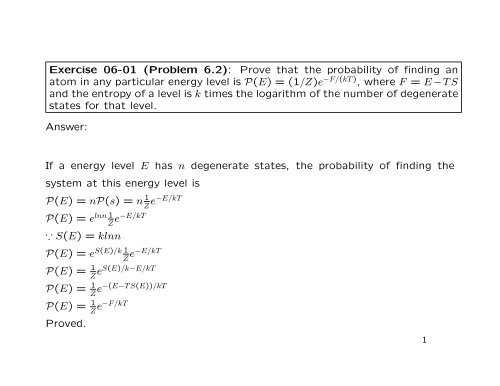
Finally for part c, this is a shot in the dark, but the average kinetic energy of helium is 3/2NKT , so if equate 3/2NKT = 1/2(torsion constant)(torsion angle)^2 and solve for it i end up getting something that doesn't make sense Likes Delta2 and PhDeezNutz Answers and Replies.
32nkt. Eint = 3/2 NkT = 3/2 nRT where n is the number of moles Each direction (x, y, and z) contributes (1/2)nRT to the internal energy This is where the equipartition of energy idea comes in – any other contribution to the energy must also contribute (1/2)nRT. Formula U = (3/2)(NkT) Where, U = Internal Energy of Monatomic Gas N = Number of Particles k = Boltzmann Constant T = Temperature Related Calculator. That is by equating (32) and (35) v rms = 3kT/m 1/2.
Problemasdegasesideales 1 12 TERMODINÁMI CA AMBIENTAL 2 1 Se lleva a cabo un experimento de Dumas en el cual se determinan las cantidades de presión, temperatura y volumen para una muestra de gas. Finally for part c, this is a shot in the dark, but the average kinetic energy of helium is 3/2NKT, so if equate 3/2NKT = 1/2 (torsion constant) (torsion angle)^2 and solve for it i end up getting something that doesn't make sense. U = (3/2)nRT = (3/2) NkT (44) Where N A = 1023 x 10 23 the Avogadro's number and k = R/N A = 138 x 10 23 T/K the Boltzmann Constant From the equations (31) and (34), we get = (3/2) kT (45) The rootmeansquare of speed v rms is defined as v rms = 1/2;.
Shop your Liquid Sci Glass now!. Monatomic ideal gas E int = (3/2)NkT = (3/2)nRT Each direction (x, y, and z) contributes (1/2)NkT to the energy This is where the equipartition of energy idea comes in any other contribution to the energy must also contribute (1/2)NkT. By signing up, you'll get thousands of stepbystep solutions to your homework questions You can also.
Presentation 1 March 11 Page 4 NKT Holding A/S / IR presentation / Interim Report 1, 12 Page 4 Highlights Q1 12 • Revenue 3531. Start from this definition, and show that for the classical limit gs >> ng >> 1 9s log WFD anlog tns ns (b)5 Consider the SackurTetrode equation for the case of ideal gas which can be written as S = Nk (5/2 log{ xh (458) **} Start from this equation and show that the energy of the ideal gas is E=3/2NKT. Dos niñas de once años, ambas con membresias en The American Mathematical Society y The European Society for Mathematical and theoretical Biology, tratan tem.
I had always thought the total kinetic energy in a system is PV = nRT, but today at school I saw someone say it was 3/2 nRT or 3/2 PV Can anyone prove this and explain why PV does not yield the kinetic energy of a system?. Our ORIGAMI is specifically designed for OEM integration It is the industrialgrade, ultracompact, modelocked, femtosecond laser that provides the lowest phase noise and timing jitter on the market. American made expertly crafted glass for a better smoking experience What are you waiting for!.
Stack Exchange network consists of 176 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers Visit Stack Exchange. My thinking was this PAd = some tiny work done by n moles of the gas, d is a tiny distance moved v = Ad = tiny volume expanded or contracted N/n = ratio between total moles. Alternate form PV = NkT where N is the number of molecules in the gas and k is the Boltzmann’s constant, k = 138x1023 J/K (Comparing the two forms gives R=NAk) All real gases approach the “ideal gas” in.
The equation of a state of an ideal gas is pV=NkT The internal energy is U= (3/2)NkT. Solved What is k, in the formula, (3/2)kT?. 3 2 NkT É(2) Substituting NkT from (2) into (1) we Þnd P = 2 3 Eth V, 2/3thelocalthermal The local pressure is equal to 2/3 the local thermal energy density Principles of Astrophysics & Cosmology Professor Jodi Cooley Multiply by 4"r2 and integrating over the volume of the star, we Þnd.
What is Nuclear Power This site focuses on nuclear power plants and nuclear energy Main purpose is to provide knowledge base not only for experienced. Looking for the definition of NKT?. My thinking was this PAd = some tiny work done by n moles of the gas, d is a tiny distance moved v = Ad = tiny volume expanded or contracted N/n = ratio between total moles.
493 where H is the classical Hamiltonian, h is Planck's constant, and the classical partition function Q is Q = hM ∫ exp ( H(q, p)/kT) dq dp This probability density expression, which must integrate to unity, contains the factor of. Presentation 1 March 11 Page 4 NKT Holding A/S / IR presentation / Interim Report 1, 12 Page 4 Highlights Q1 12 • Revenue 3531. American made expertly crafted glass for a better smoking experience What are you waiting for!.
Electrons, and another 3/2NkT to bring the ions up to the ambient temperature 122 MIXTUREOFIDEALGASANDRADIATIONIONIZATIONEFFECTS 73. Learn what the first law of thermodynamics is and how to use it. 2 Peter 23 New King James Version (NKJV) Destructive Doctrines 2 But there were also false prophets among the people, even as there will be false teachers among you, who will secretly bring in destructive heresies, even denying the Lord who bought them, and bring on themselves swift destruction 2 And many will follow their destructive ways, because of whom the way of truth will be blasphemed.
The change in internal energy of a system equals the heat added to the system plus the work done on the system (Beware of sign conventions in other books!) Note that for an ideal gas, U = (3/2)NkT, so that if the temperature does not change the internal energy cannot change. Learn what the first law of thermodynamics is and how to use it. Wed = II 9,!.
The eigenstate thermalization hypothesis (or ETH) is a set of ideas which purports to explain when and why an isolated quantum mechanical system can be accurately described using equilibrium statistical mechanicsIn particular, it is devoted to understanding how systems which are initially prepared in farfromequilibrium states can evolve in time to a state which appears to be in thermal. PV = NkT = nRT U = (3/2) NkT = (3/2)nRT Monatomic Ideal Gas Thermodynamics, Thermal Conduction, Thermal Radiation Q = kAt(∆T/L) P = eAσT4 ∆U = Qin – Wby W = P∆V ∆S = Q/T ∆Suniverse≥0 Heat Engines e = W/Qh emax = 1 (Tc/Th) QUANTITY SYMBOL UNIT OR VALUE COMMENTS. Alternate form PV = NkT where N is the number of molecules in the gas and k is the Boltzmann’s constant, k = 138x1023 J/K (Comparing the two forms gives R=NAk) All real gases approach the “ideal gas” in.
Formula U = (3/2)(NkT) Where, U = Internal Energy of Monatomic Gas N = Number of Particles k = Boltzmann Constant T = Temperature Related Calculator. Philippians 3 New Living Translation (NLT) The Priceless Value of Knowing Christ 3 Whatever happens, my dear brothers and sisters, rejoice in the Lord I never get tired of telling you these things, and I do it to safeguard your faith 2 Watch out for those dogs, those people who do evil, those mutilators who say you must be circumcised to be saved 3 For we who worship by the Spirit of. Relationships among macroscopic variables eg PV = NkT;U = 3 2 NkT 2nd Law, Curie law for paramagnets probability densities for microscopic quantities (eg velocity distribution for an ideal gas in equilibrium) End of Lecture 10 This will be our starting point for deriving thermodynamics and other things Whether it.
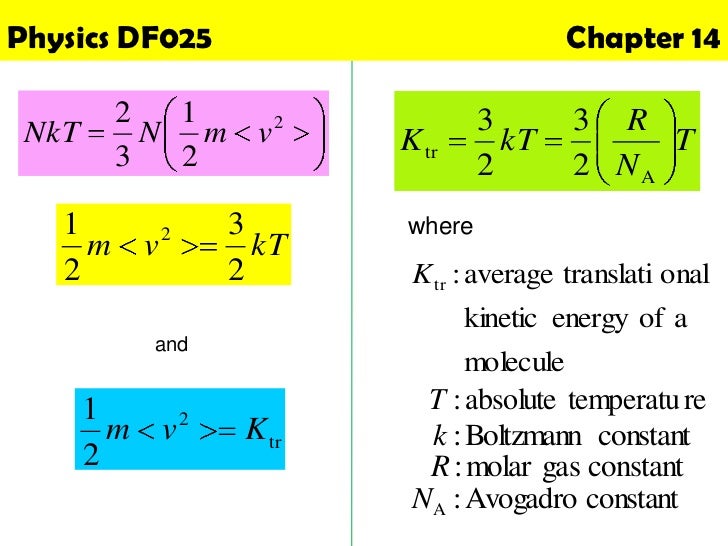
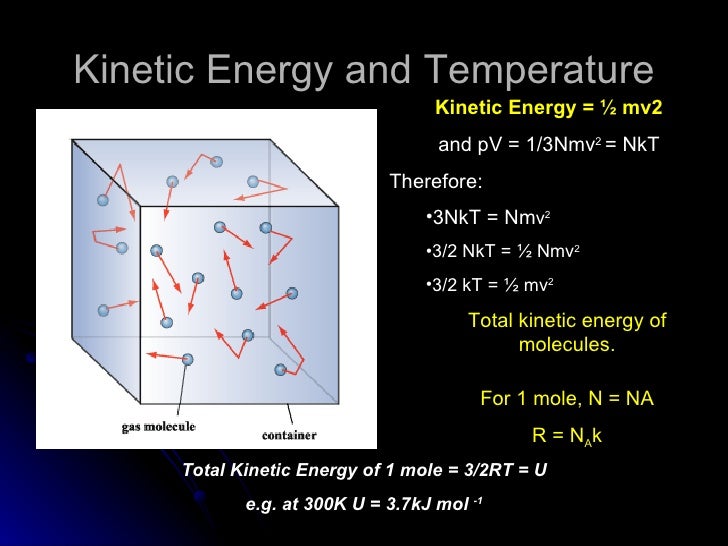
Molecular DynamicsMolecular Dynamics Chapter 21 Basic Thermodynamics Equilibrium when samples can be meaningfully used for statefunction (thermodynamic property) measurements, system is in equilibrium. Ideal Gas PV = nRT = NkT U = 3/2nRT = 3/2NkT k B = 138 X 10 –23 J/K R = 1 Latent Heat L steam = 226 X 10 6 J/kg Q = mL For reversible heat engines (Carnot) efficiency = 1 Q c/ Q h = 1 T c/ T h Q h = Q c W COP for Heat Pump = Q h / W COP for Refrigerator = Q. K tot trans = N 1 2 m ¯ v 2 = 3 2 NkT = 3 2 nRT (347) If the gas only has translational kinetic energy, this is the internal energy of the gas – this is true for ideal monoatomic gases (one atom per molecule) This tells us that the internal energy of an ideal gas depends only on the temperature The root mean square (rms) speed is the square root of the average of the squares of the.
We can actually generalize the total energy to U = 3/2 nRT for n number of moles or U = 3/2 nKT for n number of molecules Now, suppose we had a linear molecule (like CO2) or diatomic gas In this case, you can translate the molecule in three independent dimensions We get a contribution of 3/2 kT from this translational freedom. (3/2)nRT is the translational kinetic energy, and since almost all atoms are in the ground electronic state at low temperature, it is a good expression for internal energy as long as the temperature is low enough that essentially all atoms are in the electronic ground state. Solved What is k, in the formula, (3/2)kT?.
NKT's flexible and reliable solutions bring power to interconnections, hydroelectric and nuclear power plants, as well as onshore and offshore wind farms, oil and gas platforms and solar energy. 0J for the whole process Formula for heat required to change temperature of a substance Q = mC∆T. In onedimension, an ensemble of N classical particles has energy of the form E=p^2/2mkx^2/2 The average internal energy of the system at temperature T is(a)3/2NkT (b)1/2NkT (c) 3NkT.
3 2 NkT (See 2, pg 72) In fact, given the statistical de nition of ˝, the de nition of T in terms of ˝, and a quantum mechanical description of water at its triple point, one could, in principle, compute k in terms of fundamental atomic constants such as e, m e, and h if only one could solve. Behavior for ideal gas, PV=NkT, and U=(3/2)NkT “equation of state” • Q and W are not state variables they describe changes to the state of the system – Adding or subtracting Q or W moves the system from one state to another points in a {P,V,T} coordinate system – The system can be moved from one point to another via. 493 where H is the classical Hamiltonian, h is Planck's constant, and the classical partition function Q is Q = hM ∫ exp ( H(q, p)/kT) dq dp This probability density expression, which must integrate to unity, contains the factor of.
The KE=(1/2)mv2 video link is https//wwwyoutubecom/watch?v=7jqnKbeX5E This video is a very quick, all math, derivation of KE=(3/2)nRT=(3/2)PVThis is. The eigenstate thermalization hypothesis (or ETH) is a set of ideas which purports to explain when and why an isolated quantum mechanical system can be accurately described using equilibrium statistical mechanicsIn particular, it is devoted to understanding how systems which are initially prepared in farfromequilibrium states can evolve in time to a state which appears to be in thermal. 볼츠만 상수 는 기체 상수 와 아보가드로 상수 의 비이다 = / 이 상수의 단위는 엔트로피와 같으며, 오스트리아 물리학자 루트비히 볼츠만의 이름을 따서 지어졌다 거시 물리학에서 미시 물리학으로의 다리 볼츠만 상수 k는 거시 물리학과 미시 물리학 사이의 다리이다 거시적으로, 이상 기체.
I had always thought the total kinetic energy in a system is PV = nRT, but today at school I saw someone say it was 3/2 nRT or 3/2 PV Can anyone prove this and explain why PV does not yield the kinetic energy of a system?. 29 meanings for NKT abbreviations and acronyms on acronymsandslangcom The World's most comprehensive acronyms and slang dictionary!. By signing up, you'll get thousands of stepbystep solutions to your homework questions You can also.
Note that here U = 3/2NkT was used in the last step He (032 eV, how to calculate?) Ar (042 eV, how to calculate?) 1Mixing 1 mol He with 1 mol He (both at standard conditions) 2Mixing 1 mol He with 1 mol Ar (both at standard conditions) 115 Exercise Problem 337 Consider a monoatomic ideal gas that lives at a height z above sea level, so. Find out it here!. The change in internal energy of a system equals the heat added to the system plus the work done on the system (Beware of sign conventions in other books!) Note that for an ideal gas, U = (3/2)NkT, so that if the temperature does not change the internal energy cannot change.
U = 3/2NkT Formula for internal energy containing the Gas constant R U = 3/2nRT Formula for internal energy for a cyclic process ∆U = 3/2 nR∆T for each step;. Titanium Copper Alloy NKT322 of the JX Nippon Mining & Metals are introduced. Internal energy in an ideal gas We showed previously that the translational energy density per molecule is given by u˙trans = 3 2 kT where the number three represents the number of degrees of freedom associated with the kinetic.
Solution for week 9 PDFversionofsolutions 1 Vapor pressure equation a) To solve for dp dT we will begin with the ClausiusClapeyron equation derived in class dp dT = L T(V g − 0 V ‘) (1). Eint = 3/2 NkT = 3/2 nRT where n is the number of moles Each direction (x, y, and z) contributes (1/2)nRT to the internal energy This is where the equipartition of energy idea comes in – any other contribution to the energy must also contribute (1/2)nRT. Read Part 1 Equilibrium Systems The Ideal Gas Boltzmann’s Approach (The Microcanonical Ensemble) Consider a monatomic gas of ##N## noninteracting particles with mass ##m## occupying the volume ##V##.
I've already told you multiple times that big, uppercase U is the internal energy of a system And it's really everything thrown in there It's the kinetic energy of the molecules. I had always thought the total kinetic energy in a system is PV = nRT, but today at school I saw someone say it was 3/2 nRT or 3/2 PV Can anyone prove this and explain why PV does not yield the kinetic energy of a system?. = 3/2NkT =3/2 nRt N = number of molecules n = number of moles Heat Capacity and Calorimetry The temperature of an object will rise when you add heat (energy) to it The amount of heat (Q) required to raise the temperature of a system is found to be proportional to the mass (m) of the system and to the change in temperature ( T) of the system.
My thinking was this PAd = some tiny work done by n moles of the gas, d is a tiny distance moved v = Ad = tiny volume expanded or contracted N/n = ratio between total moles. Shop your Liquid Sci Glass now!. 3 2 NkT (See 2, pg 72) In fact, given the statistical de nition of ˝, the de nition of T in terms of ˝, and a quantum mechanical description of water at its triple point, one could, in principle, compute k in terms of fundamental atomic constants such as e, m e, and h if only one could solve.
What does NKT stand for?. Work Done in Basic Thermodynamic Processes Basic goal determine dE, dQ, and dW for general thermodynamics processes We now study 3 fundamental processes Constant Volume (Isochoric) A constant volume process is the vertical path dV = 0 in the PV planeup if heat is added and down if heat is removed Because dV = 0, the work done is dW = P dV = 0. The Boltzmann constant (k B or k) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas It occurs in the definitions of the kelvin and the gas constant, and in Planck's law of blackbody radiation and Boltzmann's entropy formulaThe Boltzmann constant has dimensions of energy divided by temperature.
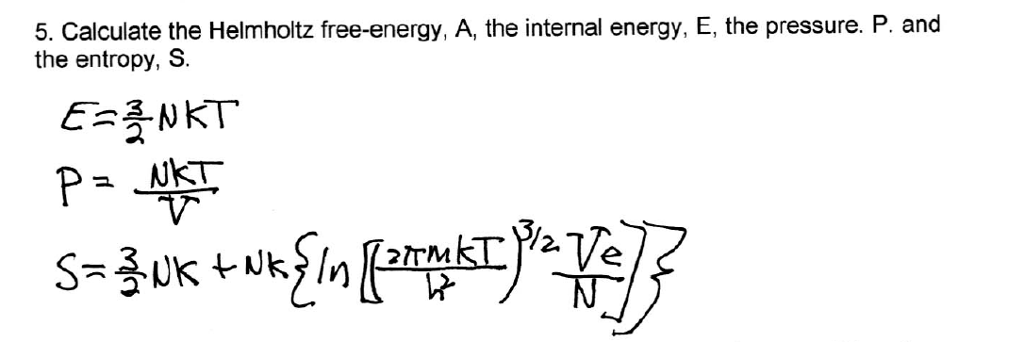
Solved Calculate The Helmholtz Free Energy A The Intern Chegg Com
Www Astro Umd Edu Richard Astro421 21 Gas Lec2 18 Pdf

The Kinetic Energy Of N Molecules Of O2 Is X Joule At 123 C Another Sample Of 02 At 27 C Has Brainly In
2

Chapter 14 The Classical Statistical Treatment Of An Ideal Gas Ppt Download

Shibata Yokoyama Em T Diagram For Solar Stellar Flares

Increased Activation Of Btla 2 2 Nkt Cells On Agr Stimulation In Vitro Download Scientific Diagram

Pdf Digital Comns 4th Edition By Simon Haykin Solns Surjit Bhowmick Academia Edu
Thermodynamics
Ppt Sol S Scrapbook Powerpoint Presentation Free Download Id

Ppt Water S Phase Diagram Powerpoint Presentation Free Download Id
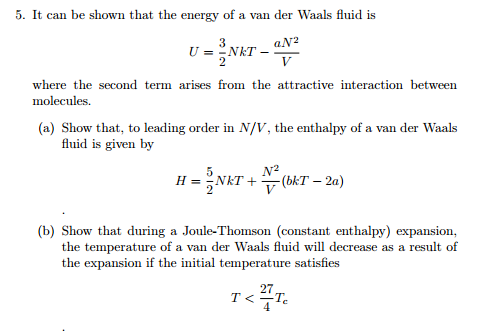
Solved It Can Be Shown That The Energy Of A Van Der Waals Chegg Com
Ppt Chapter 14 The Classical Statistical Treatment Of An Ideal Gas Powerpoint Presentation Id

Chapter 14 The Classical Statistical Treatment Of An Ideal Gas Ppt Download
Q Tbn And9gctbxjvscstlye1ujhp1azdbarzca9q9yraxbpc8uyaavm3ubl0g Usqp Cau
Q Tbn And9gcshwlqj7jl3gtmc3hjxriqmiakeixjujtbhyuckmj2g0a4chsfk Usqp Cau

Ramanujan Theta Function Wikipedia

Overview For Senrade
2

1st Law
Http Pubs Acs Org Doi Pdf 10 1021 Ed065p876
Kinetic Theory Of Gases 14 3
P210 13a
Plos One Differential Requirement For The Cd45 Splicing Regulator Hnrnpll For Accumulation Of Nkt And Conventional T Cells
Http Www Columbia Edu Itc Chemistry Chem C2407 Archive Slides Lectureslides2 Pdf
Qzhu17 Github Io Assets Pdfs Courses Phys 467 667 Lec09 Pdf

Internal Energy Ideal Gas Monatomic Diatomic Gas Nuclear Power Net
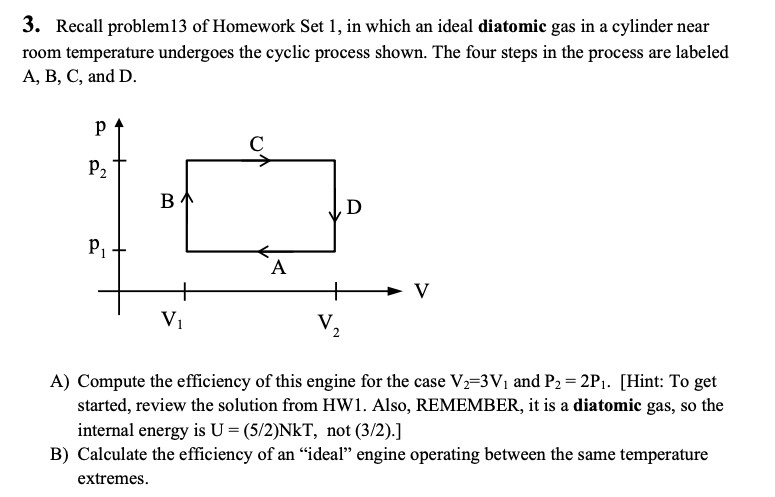
Solved 3 Recall Problem 13 Of Homework Set 1 In Which A Chegg Com
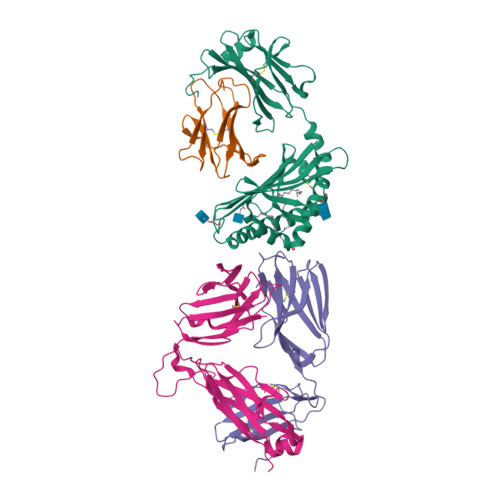
Rcsb Pdb 3tn0 Structure Of Mouse Va14vb8 2nkt Tcr Mouse Cd1d A C Galactosylceramide Complex
Http W Astro Berkeley Edu Ay216 06 Notes Ay216 06 12 Hi Thermo Pdf

Formulas To Remember Chem And Phys Flashcards Quizlet
2
1st Law

Monatomic Molecules An Overview Sciencedirect Topics
2
Http Www Physics Unlv Edu Qzhu Teaching Thermalphysics Phys467 Pdf
Www Astro Umd Edu Richard Astro421 21 Gas Lec2 18 Pdf
Http Valeriefaulknermathclub Files Wordpress Com 12 08 Assessment Project Part 1 Rubric Pdf
Http Hsiaoscu Pbworks Com F Review 2 Pdf
Scholarworks Iupui Edu Bitstream Handle 1805 Gao 18 Variance Pdf Sequence 1 Isallowed Y

Monatomic Molecules An Overview Sciencedirect Topics
2

Thermal Physics Temperature Measures The Tendency For Energy To Leave An Object Spontaneously A Measure Of The Average Kinetic Energy Of The Molecules Ppt Download
2
Wrf Model Initialization Applied To A Case Of Explosive Cyclogenesis Case In The Southern Region Of Brazil

Midterm Exam 1 College Physics Fall 07 Phys 2 Docsity
Http Www Astronomy Ohio State Edu Ryden Ast5 Ch11 Pdf
Http Www Physics Sfsu Edu Wman Phy111hw Lecture notes Chapter18 Pdf
Http Www Physics Unlv Edu Qzhu Teaching Thermalphysics Phys467 Pdf

Plot Of The Mean Electrostatic Energy U 2nkt Vs A A For The Download Scientific Diagram
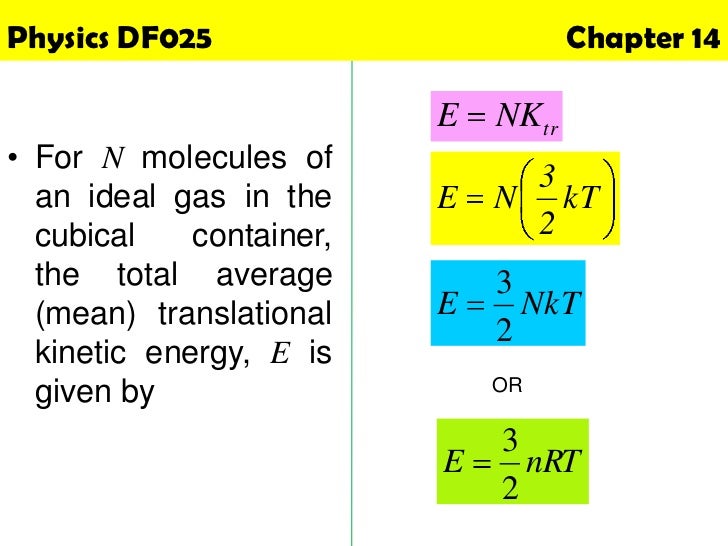
Physics Chapter 14 Kinetic Theory Of Gases
Additional Materials
2

Solved Example Problems For Physics Kinetic Theory Of Gases

Formulas To Remember Chem And Phys Flashcards Quizlet
Physics Chapter 14 Kinetic Theory Of Gases
Suli Pppl Gov 18 Course Intro to magnetiic fusion Pdf
Ijms Free Full Text Interleukin 18 In Health And Disease Html

Kinetic Theory Boundless Physics
Http Astronomy Nmsu Edu Aklypin Ast506 Thermodynamics Pdf
2

Co2 0 C O Is A Triatomic Gas Mean Kinetic Energy Of One Gm Gas Will Be N Avagadro Number K Boltzmann Constant And Molecular Weight Of Co2 44 58 5 2 Nkt 3 Nkt 7 4 Nkt

Entropy Ideal Gas

Statistical Physics Ii Phys 4240 Department Of Physics
13 5 Kinetic Theory2
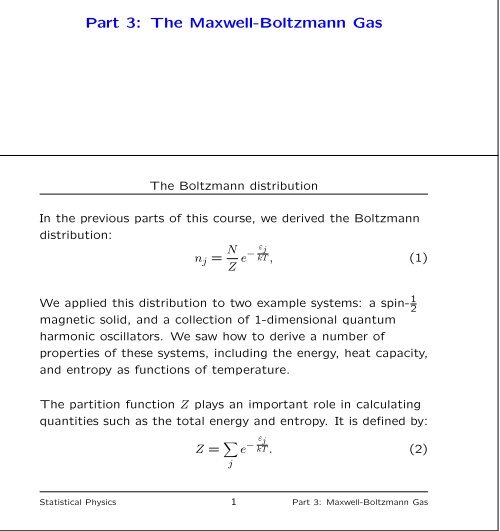
Part 3 The Maxwell Boltzmann Gas
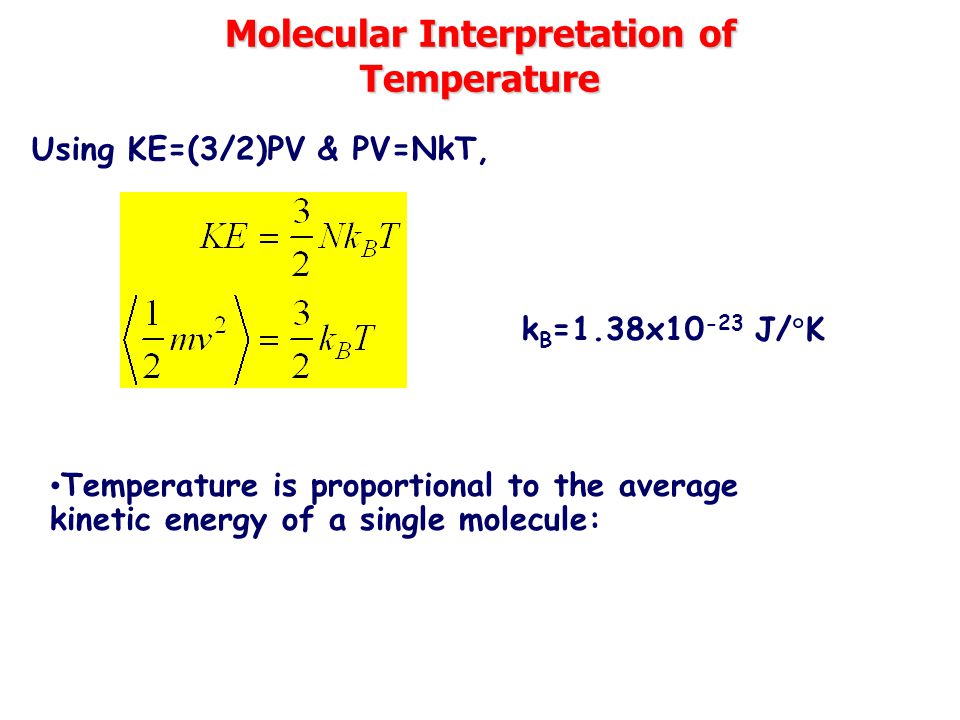
Chapter 10 Thermal Physics Temperature And Heat Ppt Video Online Download

Homework 1solution Hw 1 Solutions Homework Cube Of Ice Having Temperature Is Studocu

General Physics L02 Paths Ppt Energy Transfers Ppt Download
Http Www Gc Cuny Edu Cuny Gc Media Cuny Graduate Center Pdf Programs Physics All Master File Problem Set Statistical Mechanics June 10 To Current 05 14 Pdf
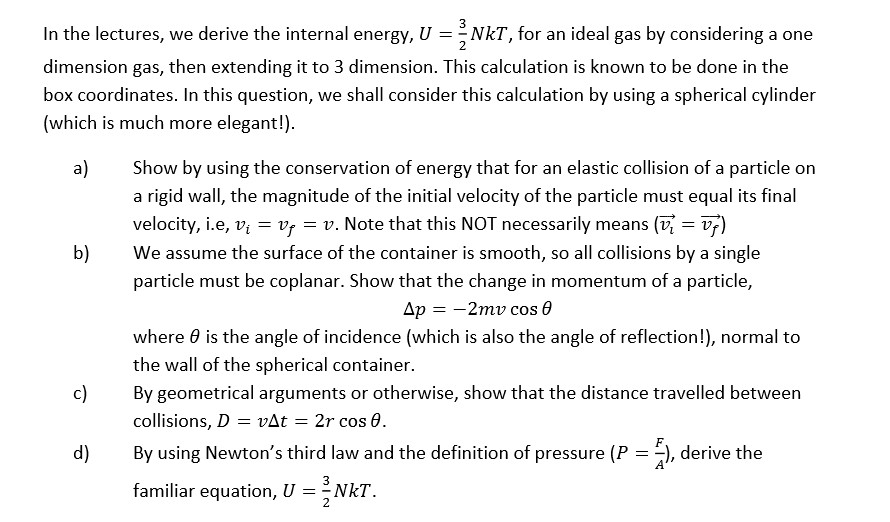
Solved In The Lectures We Derive The Internal Energy U Chegg Com

Kinetic Theory Boundless Physics
Q Tbn And9gcrjzer7vt1q66b7tppiliniux7zvndiv2alafk8pidco0mdbmaj Usqp Cau

Solved Example Problems Expression For Pressure Exerted By A Gas Kinetic Theory Of Gases Physics
2
Www Colby Edu Chemistry Pchem Notes Plnpq Pdf

At 27 C The Total Kinetic Energy Of 8 Gramshydrogen Is Times The Total Kineticenergy Of Brainly In
2
How To Find Added Thermal Heat In Monoatomic Gas Physics Forums
2
Http Home Strw Leidenuniv Nl Keller Teaching Planets 09 Planets09 E03 Pdf
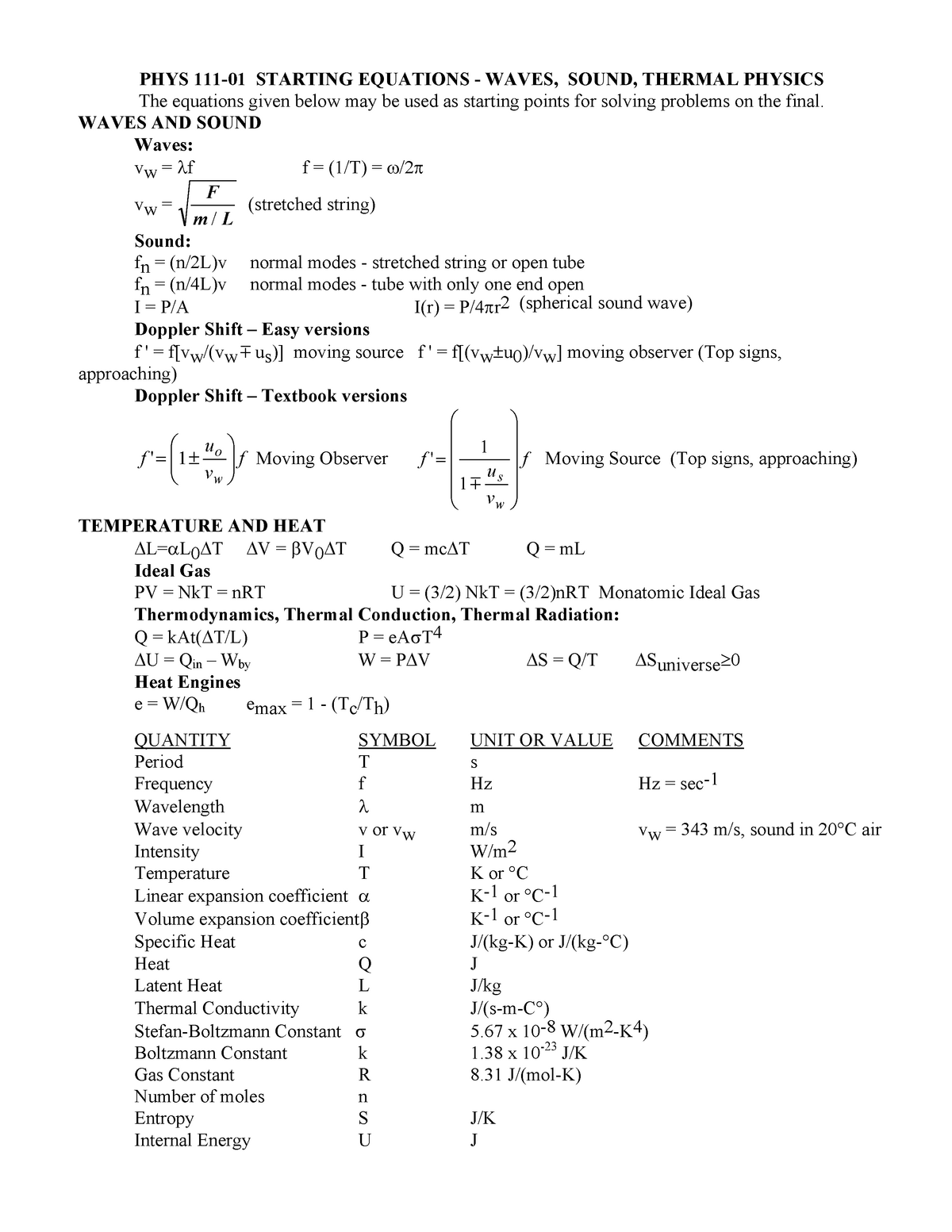
P111 F09 Equation Sheet 3 Sfsu Studocu
Suli Pppl Gov 18 Course Intro to magnetiic fusion Pdf
Q Tbn And9gctdxopxkif00upj7tdi Rty6fut5dxboprtzqzognwu Ctx 0sw Usqp Cau
Proof U 3 2 Pv Or U 3 2 Nrt Video Khan Academy

13 Matter Very Simple The Gas Laws Ppt Download

Solved Example Problems Expression For Pressure Exerted By A Gas Kinetic Theory Of Gases Physics
How To Find Added Thermal Heat In Monoatomic Gas Physics Forums
Http Courses Washington Edu Phy115a Slides Finalex14 Formulas Pdf

The Kinetic Theory Of Gases Pdf Free Download

Kinetic Theory Boundless Physics
Www Uio No Studier Emner Matnat Fys Fys4130 V09 Undervisningsmateriale Compendium Pdf
Http Www Genetics Org Cgi Data Genetics 112 Dc1 1

Chapter 13 Learning Objectives Crypt

Ct1a
2
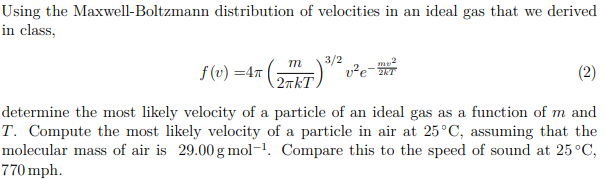
Solved Using The Maxwell Boltzmann Distribution Of Veloci Chegg Com

Derivation Kinetic Energy 3 2 Nrt Youtube



